What are stem cells?
Stem cells are undifferentiated cells, unlike the specialised cells in our body such as nerve cells, muscle cells and blood cells. They are capable of developing into various types of cells and tissues. In the human body, stem cells serve as an internal repair system during early growth and throughout life. When a stem cell divides, it potentially differentiates into another type of cell with a more specialised function, e.g. a red blood cell or a nerve cell. To put it simply, stem cells build tissues when and where the body needs them.
Stem cells can be distinguished from other types of cells by these unique properties: (1) they are unspecialised ‘immortal’ cells, capable of dividing many times over, sometimes after long periods of inactivity; (2) under certain conditions, they can be induced to differentiate into specific cell types. In the gut and bone marrow, stem cells regularly divide to replace worn out tissues. In the heart and pancreas, however, stem cells only divide under stressed conditions.
There are three major types of stem cells of the human source:
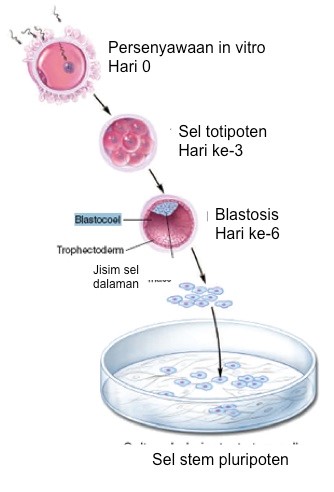
- Human embryonic stem cells (hESCs) are derived from human embryos blastocyst generated through an in vitro fertilisation (IVF) procedure for reproductive purposes (refer Figure 1). The embryos were collected from fertility clinics via informed consent of donors.
Figure 1:
Source: Winslow T. (2006)
- Adult (somatic) stem cells reside in specific tissues and normally grow to replace a particular type of tissue within its surroundings. It is essentially an undifferentiated cell found among differentiated cells in a tissue or an organ, silently in-waiting to act to repair or replace damaged or worn tissues. For example, stem cells in the bone marrow differentiate into several types of blood cells. This ability makes it possible to perform bone marrow transplant from a donor to regenerate a complete blood system in a recipient.
- In addition to the above, current technology allows the reversal of a specialised cell into an embryonic stem cell. This reprogrammed cell is called an induced pluripotent stem cell (iPSC).
What are the common terms used to describe stem cells?
Stem cells can be classified according to their developmental versatility, or ‘plasticity’. This indicates how committed a stem cell is to differentiate and become a particular type of cell.
Totipotent stem cells:
The most versatile of stem cell types, they have the potential to give rise to any and all cells or even an entire organism. When a sperm cell and an egg cell unite, a one-celled totipotent fertilised egg is formed.
Pluripotent stem cells:
These stem cells can give rise to all tissue types but cannot give rise to an entire organism (unlike totipotent stem cells). Eg: human embryo stem cell or blastocyst.
Multipotent stem cells:
These stem cells are more differentiated and can only give rise to a limited range of cells, e.g. within a tissue type.
Adult stem cells:
These are multipotent stem cells that are undifferentiated cells present in a differentiated tissue, functioning to replace dead or damaged cells.
What are the possible applications of stem cells in medicine?
Given their unique differentiation abilities, stem cells offer much potential in medicine. Use of stem cell based therapies for treatment of diseases is referred to as regenerative medicine. Regenerative medicine has often been perceived as the next pillar in healthcare, after pharmaceutical drugs, biological products and medical devices.
As clinical application of stem cells will require safe and highly efficient generation of stem cells, fundamental scientific research attempts to answer these questions:
(1) why can embryonic stem cells proliferate for a long period without differentiating, while adult stem cells cannot?;
(2) what are the factors in the human body that regulate stem cell proliferation and self-renewal?
The answers to these questions may provide a deeper understanding on how cell proliferation is regulated during normal embryonic development or during uncontrolled cell division that leads to cancer or birth defects. The information may also allow more efficient generation of stem cells in the laboratory setting to fulfil medical needs. In mouse studies, embryonic stem cells have been successfully differentiated into nerve cells and insulin-producing cells. If the technique can be successfully replicated in human, transplantation of laboratory-produced specialised cells can potentially treat diseases such as diabetes, traumatic spinal cord injury, and Parkinson’s disease.
What is the current stage of medical research on stem cells?
The history of research on adult stem cells began more than 60 years ago. Back then, two types of stem cells were discovered in the bone marrow:
(1) haematopoietic stem cells that form all kinds of blood cells in the body; and
(2) mesenchymal stem cells that can generate bone, cartilage and fat cells. In the 1990’s, scientists discovered that stem cells in the adult brain are able to differentiate into neuronal (nerve cells) and non-neuronal (astrocytes and oligodendrocytes) cell types. Haematopoietic stem cells (HSCs) remain the best-studied stem cells to date and have seen widespread clinical use.
Although human embryonic stem cells (hESCs) have the potential to provide an unlimited resource of tissue for transplantation therapies to treat many degenerative disorders, a number of challenges exist in the development of hESC-based transplantation therapies. Researchers are attempting to answer these of the questions:
- How do transplanted stem cells find their ‘niche’ area within the human body?
- How can stem cells be transplanted safely and dependably to the target tissue or organ?
- What is the long term fate of transplanted cells in the recipient’s body?
- How can transplanted cells be tracked in the recipient’s body?
Clearly, many more years of research is required before the basic research on embryonic stem cells can be translated to use in treating patients.
What are the approved uses of stem cells in Malaysia?
In Malaysia, tissue specific adult stem cells are most robust in its clinical application. Stem cells found in the bone marrow naturally makes red blood cells and white blood cells, and have been used in transplants for the past 40 years. Haematopoietic stem cells (HSCs) are used in routine bone marrow transplantations to treat blood cancers (leukaemias and lymphomas). The use of HSCs has also been coupled with administration of high-dose chemotherapy in treating other types of cancer. Other indications for HSCs include diseases involving genetic or acquired bone marrow failure, e.g. aplastic anaemia and thalassaemia.
Apart from the uses mentioned above, other uses of stem cells are considered to be experimental in nature and are only allowed for medical research purposes. Local requirements are outlined in guidelines published by the Ministry of Health Malaysia. The Ministry expects more comprehensive information in terms of safety, quality and efficacy before allowing other uses of stem cells in the Malaysian population.
What are the potential risks of stem cell treatment?
As rapid as new research findings emerge on the potential applications of stem cells, doubts have been raised on the unknown effects of treatment.
For reasons as presented in earlier sections, adult stem cells currently occupy a more established space in medical treatment. It has been generally accepted that a blood-forming stem cell in the bone marrow (haematopoietic stem cell, HSC) is unable to give rise to cells of a different nature, e.g. a nerve cell. In the field of HSC transplantation, the challenge remains to match donor HSCs to recipient to avoid immune rejection. At present, an efficient expansion of patient’s own HSCs in culture remains a top research priority to overcome this concern.
Other major concerns on stem cell therapy:
- The efficacy of stem cell therapies depends on the introduced cells arriving where they are needed to replace damaged cells. There is a risk of transplanted cells growing in unexpected ways or places, thus establishing the wrong tissue in the wrong place
- The immune system may reject and attack transplanted cells. The current transplantation protocol of using immune suppressing drugs presents unwanted side effects on recipients
- The virus used to introduce stem cell factors for reprogramming in iPSCs may cause cancer and the potential of an iPSC to form tumour has not been fully addressed.
In conclusion, a complete understanding on the potential of stem cells in medicine presents a great challenge to the medical community. However, rapid developments in genomics and proteomics can potentially widen the horizon for clinical application of stem cells. The ultimate aim is to transform stem cell therapy from a medical frontier to a medical basis one day.
Where can I get more information on stem cells?
The website http://www.nature.com/stemcells/links.html provides a comprehensive listing of stem cell resources with regards to ethics/policy/regulation, literature and blogs, educational resources, etc.
References
- National Institutes of Health, U.S. Department of Health and Human Services (March 5, 2015). Stem Cell Basics: Introduction. In Stem Cell Information. Retrieved April 14, 2015 from http://stemcells.nih.gov/info/basics/pages/basics1.aspx
- National Institutes of Health, U.S. Department of Health and Human Services (September 9, 2009). Stem Cell Information: Repairing the Nervous System with Stem Cells. Retrieved April 15, 2015 from http://stemcells.nih.gov/info/scireport/pages/2006Chapter3.aspx
- Nature Publishing Group (2015). Nature Reports: Stem Cells FAQs: What are Stem Cells? Retrieved April 14, 2015 from http://www.nature.com/stemcells/2007/0706/070614/full/stemcells.2007.12.html
- Nature Publishing Group (2015). Nature Reports: Stem Cells FAQs: How can Stem Cells Advance Medicine? Retrieved April 14, 2015 from http://www.nature.com/stemcells/2007/0706/070614/full/stemcells.2007.23.html
- Nature Publishing Group (2015). Nature Reports: Stem Cells FAQs: What are Some Risks of Stem Cell Therapies? Retrieved April 14, 2015 from http://www.nature.com/stemcells/2007/0706/070614/full/stemcells.2007.26.html
- Brown University, Division of Biology and Medicine (February 19, 2014). Courses: Stem Cell Classification. Retrieved April 14, 2015 from http:www.biomed.brown.edu/Courses/BI108/BI108_2002_Groups/pancstems/stemcell/stemcellsclassversatility.htm
- Winslow, T. How Human Embryonic Stem Cells are Derived (2006) [Image]. Retrieved April 15, 2015 from http://www.stemcells.nih.gov/info/scireport/pages/2006Chapter1.aspx
- Medical Development Division, Ministry of Health Malaysia (2009). Guidelines for Stem Cell Research and Therapy. Retrieved April 14, 2015 from http://www.moh.gov.my/images/gallery/Garispanduan/Stem_Cell/stem_cell_therapy.pdf
- Strauer, B.E. and Kornowski, R. (2003) Stem cells therapy in perspective. Circulation; 107: 929-934. Retrieved April 15, 2015 from http://www.circ.ahajournals.org/content/107/7/929.full.pdf+html
| Last Reviewed | : | 24 June 2015 |
| Writer | : | Dr. Yvonne Khoo Siew Khoon |
| Accreditor | : | Dr. Azizah bt. Ab. Ghani |