Introduction
Counterfeit medicines are found everywhere in the world ranging from random mixtures of harmful toxic substances to inactive and ineffective preparations. Some contain a declared, active ingredient and look so similar to the genuine product that they deceive health professionals as well as patients. But in every case, the source of a counterfeit medicine is unknown and its content unreliable. Counterfeit medicines are illegal in Malaysia. They can result in treatment failure or even death. Eliminating them is a considerable public health challenge.
Defination
The World Health Organization (WHO) defines counterfeits medicines as “medicines that are deliberately and fraudulently mislabelled with respect to identity and/or source. Both branded and generic products are subject to counterfeiting. Counterfeit medicines may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient or too much active ingredient, or with fake packaging“.
As for Malaysia, there is no specific definitions on counterfeits medicines, however the legislation for counterfeit products are well covered under The Trade Act. According to The Sales and Drug Act 1952: Regulation 7(1)(a), 7(1A)(a-g) of the Control of Drugs and Cosmetic Regulation (CDCR) states that all medicinal products must be registered with National Pharmaceutical Control Bureau (NPCB) Ministry of Health before being marketed. All registered medicinal products will be given a unique registration numbers.
Products with the following criteria are considered unregistered medicinal products which include the counterfeit medicinal products:-
(i) if they bear
- No registration number
- False registration number
- Registration number belonging to other products
- Registration number which has been cancelled
- No safety label hologram Meditag TM
- False hologram Meditag TM
- Ingredient which differ from the original formula during registration
- A mixture of 2 registered products
- Information on the packaging which differ from the approved version
(ii) if they contain Adulterants
Control
The Ministry of Health has imposed the use of security label hologram (picture 1) to all medicinal products since September 2005. This is to prevent and control counterfeit products assessing the Malaysian market. Medicinal product with genuine MeditagTM hologram is considered genuine and medicinal product with fake hologram or without the MeditagTM hologram is considered counterfeit and unregistered. The authenticity of the MeditagTM hologram can be examined by the public in all community pharmacies in Malaysia using Decoder MeditagTM (picture 2).

Picture 1: Meditag Hologram
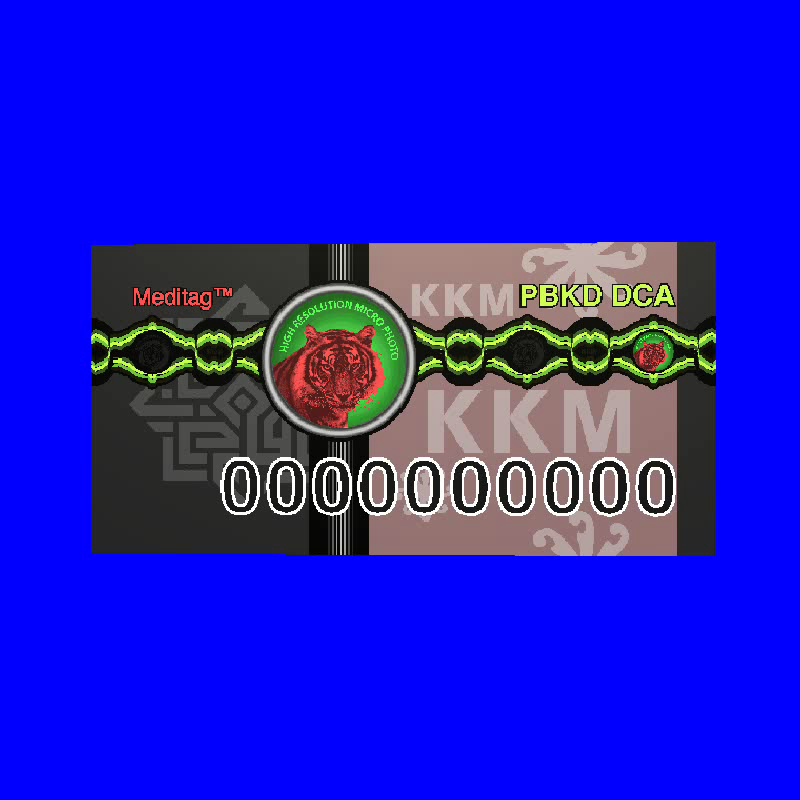
Picture 2 : Latest Version MeditagTMHologram

Picture 3: Meditag Decoder

Picture 4: Verifying the authenticity of the Meditag hologram in pharmacy
Reporting
If a medicine is suspected to be illegal or counterfeited, publics are advised to stop taking and seek medical advice should they fell unwell. They can report the incident to their pharmacist or doctor who will then inform the Ministry of Health accordingly. Alternatively they may report directly to the:
Pharmaceutical Services Division,
Ministry of Health, Malaysia,
Lot 36, Jalan Universiti,
46350 Petaling Jaya.
Tel: 03 7841 3200
Fax: 03 7968 2251 or
email to pharmacy1@moh.gov.my.
Public can visit the Pharmaceutical Services Division official web sites, www.pharmacy.gov.my for further information.
| Last reviewed | : | 23 April 2014 |
| Writer | : | Mohammad Rizalmazli bin Salim |
| Accreditor/Reviewer | : | Mazlan bin Ismail |







